About 11 horrific months have passed since the emergence of the Coronavirus in December 2019 and the announcement of a state of emergency by the World Health Organization (WHO) in January 2020.
The Coronavirus disease (COVID-19) pandemic, with very bitter and painful consequences for the world economy and health, continues to spread rapidly.
During this challenging period and until the publication of this report, according to the official statistics of governments, about 46,823,758 people have been infected and more than 1,205,325 people have died.
Writer : Fereshteh Hashemi
The world now has a strong understanding that in this pandemic, all countries are interconnected and even one virus carrier could be a threat to the whole world!
This horrific epidemic has once again forced the World Health Organization (WHO) which has spent past years with peace of mind for resolving the Ebola and SARS epidemic to go to war with the Coronavirus in a research campaign entitled the Solidarity Clinical Trial for Covid-19 Treatments.
Hopes are now pinned on the achievements of this study , while there are many questions about this insidious and complicated, sometimes “head down and quiet” and sometimes “ruthless and murderous” virus.

Professor John-Arne Røttingen is the Chair of the Executive Group of the International Steering Committee for the Solidarity Clinical Trial for Covid-19 Treatments of the World Health Organization (WHO).
He is the Chief Executive of the Research Council of Norway and Adjunct Professor at the Department of Global Health and Population, Harvard T.H. Chan School of Public Health.
Røttingen was until recently the founding Chief Executive Officer of CEPI (Coalition for Epidemic Preparedness Innovations ) ; Executive Director of Infection Control and Environmental Health at the Norwegian Institute of Public Health; and Professor of Health Policy at the Department of Health Management and Health Economics, Institute of Health and Society, University of Oslo.
He has been Chief Executive of the Norwegian Knowledge Centre for the Health Services; Oxford Scholar at Wadham College; Fulbright Fellow at Harvard Kennedy School; Chair of the Board of the Alliance for Health Policy and Systems Research; and Chair of the Consultative Expert Working Group on Research and Development: Financing and Coordination (CEWG), World Health Organization
He received his MD and PhD from the University of Oslo, an MSc from Oxford University and an MPA from Harvard University.
He has been working on innovation and access to medicines and vaccines, including policy issues and global governance of antimicrobial resistance, epidemics and health security.
Røttingen in an interview with Iran Science Watch (ISW) answers to questions about the achievements of the Solidarity Clinical Trial for COVID-19 Treatments, the COVID-19 vaccines and as well as the inevitable facts of the COVID-19 pandemic.

Photo: AP /Alessandra Tarantino
Despite warnings from scientists about the possibility of crisis of an infectious disease epidemic in the future, was the World Health Organization (WHO) well prepared and launched its research campaign on time?
The independent Global Preparedness Monitoring Board argued in their report one year ago that the international health security system was not fit for purpose to handle a pandemic.They were right. We were not prepared sufficiently. Not even the scientists that were closest to this sort of prediction were prepared.
I believe we all can agree that the world, individual countries as well as the WHO should have been better prepared. Of course, first and foremost we expected a flu pandemic, and I think we would have been better prepared if we had the flu pandemic.
The WHO has the necessary structures, but should have invested more in capacity. On the R&D side, the WHO acted quickly through the R&D Blueprint mechanism.
The WHO convened an early global R&D summit in mid February, and started planning of the Solidarity trial. The initiation was therefore quite quick and the trial.
I think we are now in a good position of international collaboration and we are in a better position now than we were with Ebola and the world has never been better prepared than in early 2020!

Photo : AP/ John Minchillo
Despite the initiation and implementation of more than 180 clinical trial studies to achieve signs of COVID-19 treatment, how necessary and important was the creation of the Solidarity Clinical Trial for COVID-19 Treatments?
Solidarity Clinical Trial provides us with a great way of achieving fast-track data generation in a large-scale mega-trial. countries with more capacity can collect much more detailed clinical trial data. Randomized trials are really important but due to little participation and small samples cannot answer important questions about epidemics of this magnitude and complexity.

Photo: Katrine Gramnæs
How do you assess the participation of countries in the Solidarity Clinical Trial for COVID-19 Treatments ? What are the most important deterrents?
Currently there are 30 countries that are enrolling patients into the Solidarity Clinical Trial for COVID-19 Treatments, and 15 more countries are ready to start. Countries in all of the six WHO regions of the world are participating.Over 12 000 patients had been recruited in 500 participating hospitals worldwide.
Despite the difficult obstacles facing the study, including the formation of an international alliance to research, budget, design, conduct and complete clinical trials, provide the drugs needed to test the effect, organize the flow of information, closely monitor the proper conduct of the study, success in determining the final treatment strategy for COVID-19 definitely needs to increase the participation of countries.
There has been a strong and clear interest in many countries for participating in the Solidarity trial. The main barrier has been to sort out the formal agreements between the national ministries of health and the WHO, which in general has gone smoothly, as well as to get national clinical trial regulatory and ethics approval. The latter has proven to be more challenging in some jurisdictions, and has delayed the start of the trial in some countries. It is more challenging to run a multi-country trial across more than 20 countries if individual agencies and committees want to diverge from the global core protocol.
Photo: Penny Stephens/Western Health
What important factors will contribute to the success of countries in attracting patient participation?
To recruit many patients fast the trials will need to be pragmatic and rely to a large extent on routine data collection so that local hospital doctors (investigators) are able to enroll and follow up patients in a situation with high case load and stretched capacities. Both the Solidarity and Recovery trials have those characteristics. In addition, it is helpful if the recruiting hospitals have experience and systems in place for conducting clinical trials. If offered, patients seem to be willing to participate, so it should be possible to recruit a high proportion of patients admitted to hospital into such pragmatic trials.

With the number of registered patients, can you hope for a definitive cure for COVID-19 or should the number of patients be much higher than the existing number?
As yet there has not emerged any “wonder-cure” for COVID-19. Only the steroid dexamethasone has so far been proven to reduce mortality robustly. Experience indicates that to document effects on mortality there needs to be at least 1000 and probably around 2000 patients receiving the treatment one is evaluating.
The way I see it is that instead of just using drugs through sort of experimental use or compassionate use, we need to have evidence on whether or not they work. This is a way to give patients the best available treatments, but under a mechanism where we can actually learn from it.
Even if we can reduce the proportion of patients that need a ventilator by, say, 20%, that could have a huge impact on our national healthcare systems, and really improve the situation a lot.
I believe when there is a new disease and there are no documented effective treatments, it is very important to learn as quickly as possible whether established drugs can be repurposed for the new disease, like COVID-19. Clear documentation of both effects and no-effects is therefore important, and a trial that can do so robustly and with quality is definitely a success.

Photo: China Daily / via Reuters
One of the most important barriers to participation in clinical trials for COVID-19 Treatments is their concern about the side effects of the drugs. How has this study addressed the concerns?
The World Health Organization (WHO) study uses global rules, care standards, and entry-exit criteria to treat COVID-19. In this study, we will temporarily analyze the results and opinions and decisions will depend on the clinical effectiveness of the drugs we are testing. If the side effects of the drugs are high, we stop them immediately.
In general, we have not experienced many concerns about potential side effects of the study medications. Rather, patients have wanted to take part in the study to get access to potentially effective drugs. The challenge has therefore in some settings rather been that patients and their local doctors have started on one of the study drugs, in particular hydroxychloroquine, before they are admitted to hospitals and therefore has not been able to participate in the trial.

The WHO has announced the results of the first four treatment arms. How are new treatment arms selected?
The Solidarity Clinical Trial for COVID-19 Treatments is evaluating the effect of drugs on 3 important outcomes in COVID-19 patients: mortality, need for assisted ventilation and duration of hospital stay.
This study published interim results on 15 October 2020. It found that all 4 treatments evaluated (remdesivir, hydroxychloroquine, lopinavir/ritonavir and interferon) had little or no effect on overall mortality, initiation of ventilation and duration of hospital stay in hospitalized patients.
The Solidarity Trial is considering evaluating other treatments, to continue the search for effective COVID-19 therapeutics.
We have appointed an independent data and safety monitoring committee, and they will be the only ones who can do interim analyses, look at the data and advise in terms of stopping arms as well as stopping the trial overall.
If we can really speed up recruitment — and that’s why we want to do this at scale in many countries — there will definitely be opportunities to increase the number of arms in the trial.
Iran has surpassed other countries in patient registration. What has played the most important role in Iran’s success?
Iran has been very active and strong at recruiting patients.This seems, from the outside, to be related to a strong national leadership and coordination for the Solidarity trial, and to establish research capacities across many of the hospitals. It has been great to collaborate with Dr. Reza Malekzadeh who is serving on the trial executive group and international steering committee.
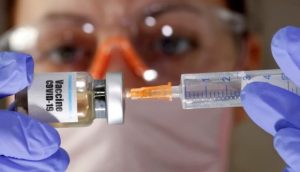
Phpto : Reuters / Dado Ruvic
Dozens of volunteer vaccines against SARS-CoV-2 under development worldwide. Great challenges still remain in the development of vaccine. What are the most important of these challenges?
The WHO is working in collaboration with scientists, business, and global health organizations through the ACT Accelerator to speed up the pandemic response.
The WHO has also provided guidance by outlining key criteria for the ethical acceptability of COVID-19 human challenge studies.
Previous work to develop a vaccine against the coronavirus diseases SARS and MERS established knowledge about the structure and function of Coronavirus which accelerated development during early 2020 of varied technology platforms for a COVID‑19 vaccine.
I believe that safe vaccine is the only key solution to controlling infectious disease,but it is a long-term solution. The challenges of developing a safe vaccine are more numerous and complex than the challenges of developing a drug for the definitive treatment of COVID-19 disease.
One of the big challenges is safety . COVID-19 vaccine should be safe for all of populations. we will need a population with sufficiently high risks of actually getting COVID-19 and not a population with a high level of immunity.
Another major challenge is the severity of COVID-19 disease in patients with diabetes, cardiovascular disease, chronic obstructive pulmonary disease ,hypertension, and especially obesity: Whether immunization with the vaccine promotes diabetes? Can vaccines have the same effect on all obese people? The challenge with elderly populations is that they typically have weaker immune responses.we will want to see whether these vaccines work in elderly individuals and people with comorbidities.
Also, the volume of vaccine production and its fair distribution is the next challenge after its development.
Given that the development of a drug or vaccine for Coronavirus is a long-term solution, what prospect do you see for COVID-19?
We all need to be very patient and adhere to health protocols. We have no choice but to use protective equipment, especially masks, wash our hands regularly, observe social distancing and stay away from communities. Otherwise, it is almost impossible to control this epidemic and this situation will continue!
Your email address will not be published. Required fields are marked *